Clinical Genetic Testing Services
Tumour Based Comprehensive Genomic Profiling
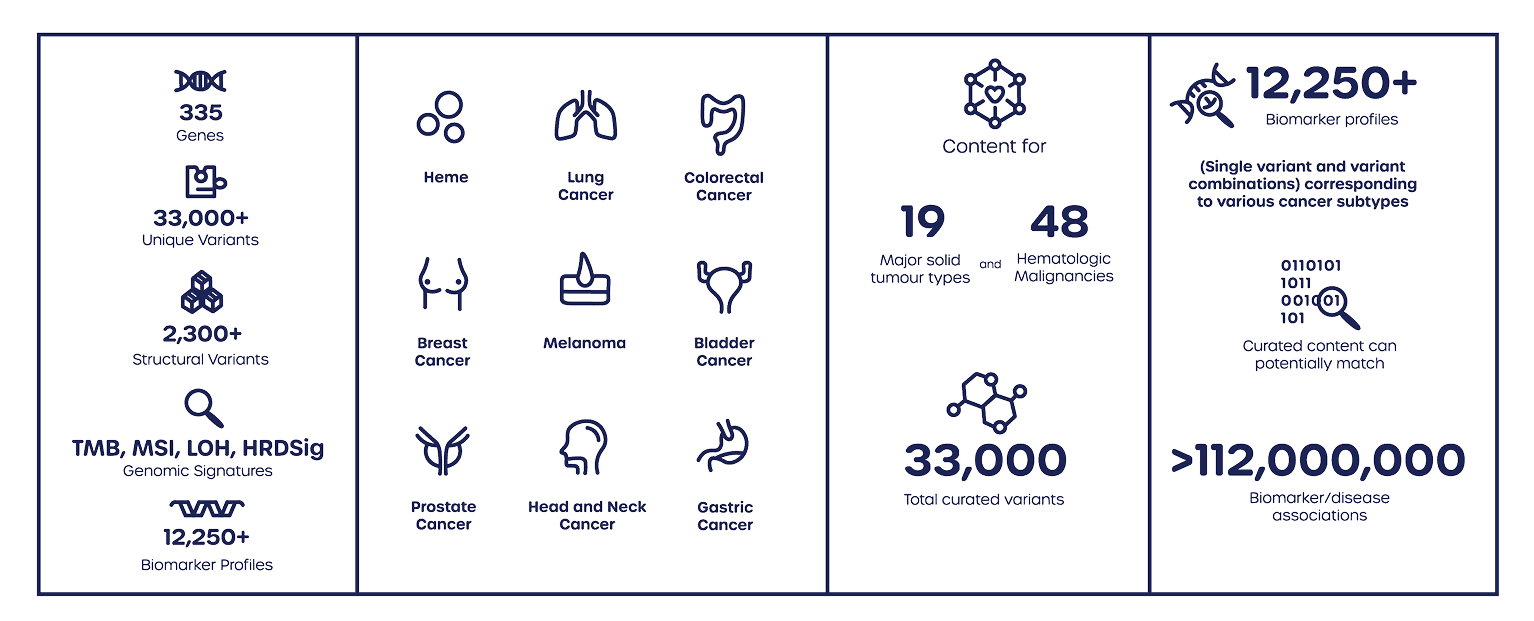
This Tumour based CGP test provides a 335 gene pan-cancer, next-generation sequencing approach to identify clinically actionable mutations. Tumour Tissue CGP can also accelerate biomarker-driven clinical trials and companion diagnostic (CDx) development with a CGP solution. This comprehensive panel is designed to align with the FoundationOne® CDx framework and features a pan-cancer HRD signature informed by Foundation Medicine’s extensive database of over 600,000 tumour genomic profiles.
Gene Content
335 pan-cancer genes panel aligned with the FoundationOne® CDx framework
Genomic Signatures
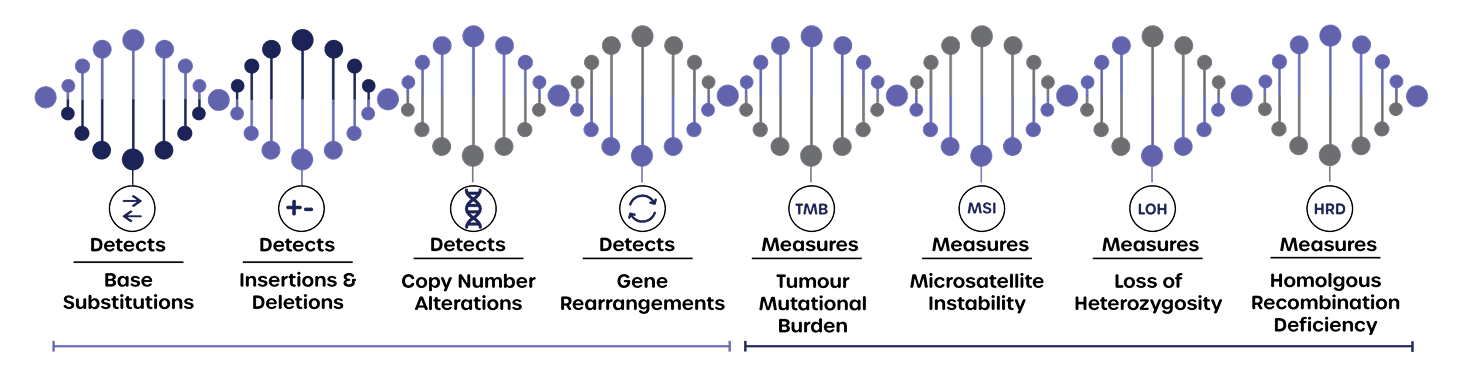
Tumour Mutational Burden (TMB), Microsatellite instability (MSI), Homologous Recombination Deficiency (HRD) and genomic loss of heterozygosity (gLOH).
Detected Mutations
CNVs, Insertions, deletions, rearrangements and base substitutions
Bioinformatic Processing
Thoroughly validated bioinformatics pipeline via FoundationOne® Analysis Platform based on 800+ peer reviewed publications and 1.3 million+ clinical samples reported. Tertiary analysis perform using the Roche CE-IVD Navify Mutation Profiler.
Comprehensive Genomic Profiling
Technology
OncoCGP offers a validated, automated Avenio Tumour Tissue CGP Kit V2* workflow which comprises of 335 genes. The workflow incorporates efficient NGS workflows, up-to-date validated bioinformatics algorithms, and successful incorporation of a pan-tumour HRD signature (HRDsig). Backed by the trusted expertise and proven technology of Roche and Foundation Medicine, Inc.Why order tumour based comprehensive Genomic Profiling to accelerate biomarker-driven clinical trial and companion diagnostics (CDx) development
- Enhance Patient Stratification
Better inform clinical trial recruitment by enrolling patients based on precise molecular profiles. - Accelerate Biomarker Discovery
Rapidly identify novel genomic alterations that may serve as predictive or prognostic biomarkers across diverse tumour types. - Enable Development of Companion Diagnostics (CDx)
Generate robust, clinically relevant genomic data to support CDx validation and regulatory approval pathways.
* The research use only assay was validated and its performance characteristics determined under ISO15189 accreditation by the Irish National Accreditation Board. The laboratory is ISO15189, CAP and CLIA certified to perform high-complexity testing. Any decisions related to patient care and treatment choices should be based on the independent judgement of the treating physician.